|
|
Is fuel efficiency really what we need most desperately?
I say that what we
really need is a car that can be shot when it breaks down.
Russell Baker
Solid oxide fuel
cells (SOFCs) are an important technology for efficient utilization of
hydrogen. Steam electrolyzers and ceramic membranes are closely related technologies
that can enable highly efficient production and utilization of hydrogen. SOFCs are
nearing commercial viability for some applications, but fundamental challenges remain,
especially reducing operating temperature and improving the stability and fuel flexibility
of the anode. Operating temperature is important not only for making the technology viable,
reducing the cost of high-temperature components, improving the stability of metallic
interconnects, and easing high-temperature seal issues, but for enabling new SOFC applications
such as transportation and portable generation. Anode alternatives to metallic Ni are needed to
improve stability during redox cycling and during electrolysis at high steam contents, and
for enabling highly efficient hydrogen production from natural gas
|
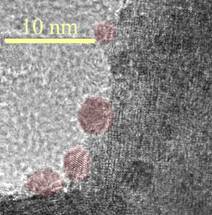 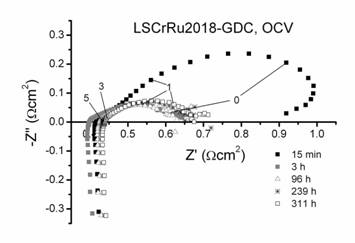
Figure 1.(a) High-resolution electron microscope image showing a portion of a La0.8Sr0.2Cr0.82Ru0.18O3
particle with Ru nano-clusters that nucleated upon reduction in H2
at 800C for 45 h. (b) Electrochemical impedance spectra obtained at various
times from a SOFC with LSCrRu-GDC anode at 800C, showing a rapid decrease in
polarization resistance that coincided with the nucleation of Ru
nano-clusters.
|
The basic premise of this project is that significantly enhanced electronic and ionic
transport properties of nano-scale oxides, combined with the high surface area
of nano-porous materials, offer an opportunity to address electrode
polarization and conductivity issues limiting low-temperature SOFC performance.
We have already developed new approaches to predict, characterize, and
understand low-temperature transport processes in new nano-scale materials that
are important for SOFCs. For instance. new fabrication methods have been
developed to produce nano-scale materials that are stable under low-temperature
SOFC conditions. Figure 1 illustrates a novel method that was developed for
producing nano-scale catalysts on SOFC anodes during operation, avoiding
coarsening that would normally occur during ceramic processing, and the
polarization resistance reduction that results. Given the obvious questions regarding
the stability of nano-materials, even at reduced SOFC operating temperatures,
nano-material stability is being studied both experimentally and by modeling.
Recent Publications
- Nucleation of nanometer-scale electrocatalyst particles in solid oxide fuel
cell anodes,
B.D. Madsen, W. Kobsiriphat, Y. Wang, L.D. Marks, and S.A. Barnett,
Journal of Power Sources 166, 64 (2007)
-
Electron microscopy study of novel Ru doped La0.8Sr0.2CrO3 as anode materials
for Solid Oxide Fuel Cells (SOFCs),
Y. Wang, B.D. Madsen, W. Kobsiriphat, S.A. Barnett, and L.D. Marks,
Microscopy and Microanalysis 13, 100 (2007)
- La0.8Sr0.2Cr1-xRuxO3-Gd0.1Ce0.9O1.95 Solid Oxide Fuel Cell Anodes: Ru Precipitation And Electrochemical
Performance,
W. Kobsiriphat, B. Madsen, Y. Wang, L.D. Marks, and S.A. Barnett,
Solid State Ionics, 180, 252, (2009)
|
 Solid Oxide Fuel Cells
Solid Oxide Fuel Cells
 Solid Oxide Fuel Cells
Solid Oxide Fuel Cells